You need to keep metal safe from galvanic corrosion. This helps you avoid big damage and expensive repairs. Galvanic corrosion happens when two metals touch. Moisture makes them react together. This can make bridges, ships, and cars weaker. Many companies lose a lot of money every year because of corrosion. The total cost for fixing and keeping things working can be $875 billion each year. In the maritime world, almost one third of ship repairs are due to this problem. Fixing just one broken bridge can cost millions. If you work with metal, it is essential to find and stop this problem early.
Key Takeaways
- Galvanic corrosion happens when two different metals touch. If there is moisture, the weaker metal rusts faster.
- It is important to check metal structures often. Look at them at least once a year. This is extra important in wet or salty places.
- Use metals that work well together to lower the risk. Metals close in the galvanic series are safer to use together.
- Put on protective coatings and treatments to stop moisture and air. This helps keep rust from starting.
- Use insulation like gaskets and non-conductive washers. These keep metals apart and stop electricity from flowing.
- Act early. Fix small corrosion problems right away. This stops them from turning into bigger, expensive problems.
- Control the area around metal structures. Keep it dry and make sure water can drain away. This lowers the chance of corrosion.
- Teach your team about stopping corrosion. Training helps everyone find problems early and take care of metal structures well.
Table of Contents
What Is Galvanic Corrosion
Causes of Galvanic Corrosion
Galvanic corrosion starts when two different metals touch and get wet. This can happen with rain or saltwater. People also call it bimetallic corrosion. It happens most when metals with different properties are together. One metal turns into the anode and rusts faster. The other metal, called the cathode, does not rust.
- Galvanic corrosion is a faster kind of corrosion. It happens when two metals with different activities touch each other.
- It is a process where one metal rusts more when touching another metal, and water is present.
- Galvanic corrosion does not spread everywhere. It happens where the two metals meet.
- Pitting corrosion makes small holes, but galvanic corrosion wears away the more active metal.
Three things must happen for galvanic corrosion to start:
- You need two different metals.
- The metals must touch each other.
- There must be water or another liquid.
When these things happen, the less noble metal, called the anode, will rust. The more noble metal, called the cathode, stays safe.
Why Galvanic Corrosion Matters
Galvanic corrosion is a big problem for people who work with metal. It can make metal parts wear out fast. This can make bridges, pipes, ships, and cars weaker. If you do not fix galvanic corrosion, things can break or fall apart. In the 1950s and 60s, many homes had leaks. This happened because copper pipes touched steel pipes and rusted fast. The Silver Bridge fell in 1967, and corrosion was a big reason.
Note: Galvanic corrosion does not just cost money to fix. It can also be dangerous, break equipment, and hurt the environment by letting metal pieces out.
Here is a simple table that shows the main parts in this process:
| Element | Description |
|---|---|
| Anode | The metal that rusts and loses material |
| Cathode | The metal that stays safe |
| Electrolyte | The liquid, like water, that lets ions move |
| Return Current Path | The metal link between the two metals |
If you do not stop galvanic corrosion, the metal can get weak. This can make bridges, pipes, and ships unsafe. It also costs more to fix and can make things unsafe.
Common Scenarios in Metal Structures
Galvanic corrosion happens in many places. Here are some examples:
- Steel pipe nipples in brass or copper fittings can rust fast and break in a few years.
- Copper in water can stick to steel and cause hidden rust. You may not see it until there is big damage.
- Using ACQ-treated wood with zinc or steel nails in wet places can cause the nailsto rust quickly.
- Aluminum road signs on ACQ-treated posts have broken in less than two years sometimes.
Some jobs have a higher risk, like oil and marine work, plumbing, cars, and HVAC. These jobs use different metals together and often get wet, so galvanic corrosion happens more.
Tip: You can lower galvanic corrosion by using dielectric unions, putting on coatings, or picking metals that work well together.
Identify Galvanic Corrosion

It is important to find galvanic corrosion early. This helps you keep metal safe and avoid spending a lot of money. You can look for problems with your eyes or use special tools. These checks help you find issues before they get worse.
Visual Signs
Discoloration and Pitting
You can spot galvanic corrosion by looking at the metal. Watch for these signs:
- Rust marks, like orange or brown spots, are where water sits.
- Color changes or strange stains on the metal.
- Small holes or pits that look like tiny craters.
- Paint or coatings that peel or flake off.
- Little cracks or splits in the metal.
These signs show up where different metals touch or where water gathers. Pitting is a big clue. It looks like deep, small holes which means the metal is being eaten away.
| Assessment Method | Description |
|---|---|
| Visual Inspection | Look closely at the metal or use a magnifying glass to spot pits and stains. |
| Measuring Size, Shape, and Density | Measure pits to see how deep and wide they are. |
| Pitting Corrosion Evaluation | Use standard charts to rate pits by their size and number. |
Tip: If you see paint peeling or color changes near joints or bolts, check under the surface for hidden corrosion.
Surface Damage Patterns
Damage from galvanic corrosion often has a pattern. You might see:
- Damage where two metals meet.
- More damage on the anode, which is less noble.
- Worse corrosion occurs where water or salt collects.
These patterns help you know it is galvanic corrosion, not another kind of rust. The damage starts where different metals touch and spreads from there.
High-Risk Areas
Dissimilar Metals in Contact
Always check places where different metals touch each other. Galvanic corrosion happens fastest when this occurs. For example, stainless steel bolts in aluminum panels or copper pipes joined to steel can cause problems. The anode will rust faster, especially if the metals are very different.
| Factor | Description |
|---|---|
| Dissimilar Metals | When two different metals touch, one corrodes faster (the anode), while the other is protected (the cathode). |
| Presence of Electrolyte | Moisture or saltwater acts as an electrolyte, allowing corrosion to start. |
| Corrosion Mechanism | The electrical difference between metals drives the reaction, much like a battery. |
Presence of Electrolytes
Moisture is needed for galvanic corrosion to happen. Places that stay wet, like flat spots where water pools or areas with rain and salt spray, are at higher risk. Saltwater, high humidity, and pollution make corrosion happen faster. Pay extra attention to places near the ocean, coast, or where the weather changes a lot.
- Wet or marine places are more likely to have galvanic corrosion.
- High humidity, saltwater, and pollution make it worse.
- The metals you use and their sizes also change how fast corrosion happens.
Note: If there is no moisture or other electrolyte, galvanic corrosion cannot start, even if different metals touch.
Inspection Methods
Routine Checks
You should check for corrosion often to catch it early. Most metal structures need to be checked at least once a year. If your equipment is near the ocean or in a tough place, check more often. During these checks:
- Look for rust, pits, or peeling paint.
- Pay close attention to joints, bolts, and where metals meet.
- Check places where water collects or does not drain.
Tip: Checking often helps you find problems before they get bad. Acting early is the best way to stop galvanic corrosion.
Advanced Testing
Sometimes, you need more than just looking. Special tools can help you measure and watch for corrosion:
| Inspection Method | Description | Applications |
|---|---|---|
| Galvanic Monitoring | Measures the electrical potential between metals in an electrolyte to estimate corrosion rate. | Used on ships, offshore platforms, pipelines, and systems with cathodic protection. |
| Ultrasonic Thickness Measurements | Uses sound waves to measure how much metal remains, detecting hidden corrosion and erosion. | Provides data for asset integrity in many industries. |
| Electrochemical Impedance Spectroscopy | Tests how well a coating resists electrical flow, showing the rate of corrosion and coating quality. | Used widely for monitoring corrosion in industrial settings. |
These tools help you find hidden corrosion and see how fast it spreads. They are very helpful for big structures, ships, and pipelines.
Regular checks, both simple and advanced, are very important in metal work. They help you find early signs of galvanic corrosion and fix them before things get worse. By staying alert and using the right checks, you can keep your metal safe.
Steps for Preventing Galvanic Corrosion
Material Selection in Machining
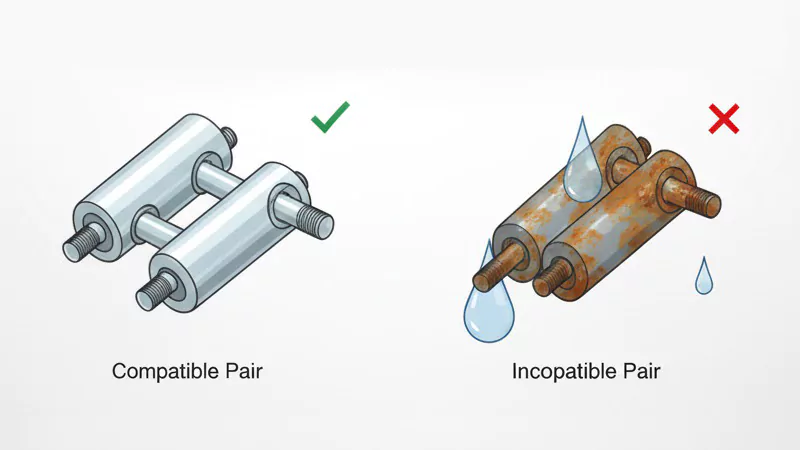
Compatible Metals
Picking the right metals helps your structures last longer. If you use metals that work well together, you lower the chance of galvanic corrosion. Metals close together in the galvanic series are safer to use together. For example, stainless steel and some aluminum alloys can be a good match. Engineers use the galvanic series to help them choose metals that will not react badly. This careful choice stops one metal from rusting faster than the other.
Here is a table with some smart ways to pick materials:
| Strategy | Description |
|---|---|
| Choose compatible metals | Pick metals with similar properties to lower corrosion risk. |
| Use non-metallic fasteners | Try using non-metal parts to join different metals. |
| Apply protective coatings | Put coatings on metals to keep them safe from corrosion. |
You can also try these ideas:
- Pick metals with similar electrical charges.
- Use alloys that do not rust easily in your area.
- Use non-metal parts to join different metals.
- Think about the galvanic series when picking important parts.
- Choose materials that make strong, protective layers.
Choosing the right metals is one of the best ways to stop corrosion before it starts.
Role of Machining in Precision and Surface Quality
Machining helps your metal parts fight corrosion. When you use precise machining, you get smooth surfaces and tight fits. This keeps water and dirt out. A smooth surface gives water fewer places to hide. This makes it harder for galvanic corrosion to start at joints or edges.
Machining also lets you control how thick and smooth each part is. You can make sure stainless steel fits tightly with other metals. This stops water from getting into small spaces. Good machining helps you avoid rough spots that can get weak. When you use good materials and careful machining, your structures last longer.
Insulation and Barriers
Gaskets and Coatings
Insulation and barriers stop metals from touching each other. This is a simple way to stop corrosion. Gaskets, washers, and special coatings act like shields between metals. Non-conductive washers and gaskets are very helpful. They keep metals apart and stop electricity from flowing.
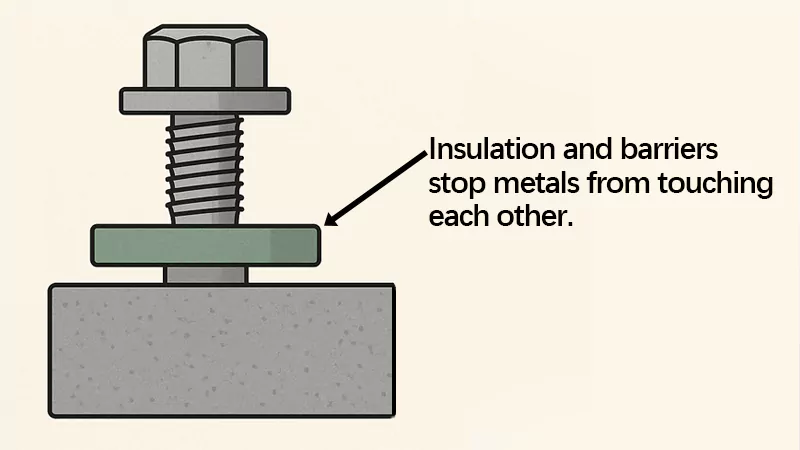
Modern coatings also help by blocking water and other liquids. Paint can make a barrier that slows down corrosion. But paint alone cannot always stop electricity. If the paint chips or wears off, the metal can still rust. That is why you should use both gaskets and coatings for the best results.
Paint can help protect materials by keeping out water and slowing down corrosion. But it cannot fully stop electricity between different metals. If paint chips or wears off, metal can rust again.
You can also use sacrificial anodes as a shield. These are metals that rust on purpose to protect the main part. For example, zinc can be used to protect steel. The zinc will rust first and save the steel.
Surface Preparation Techniques
Getting the surface ready is also important. Before you add coatings or gaskets, clean the metal well. This means taking off dirt, oil, and old paint. A clean surface helps coatings stick better and last longer. You can use sandblasting, grinding, or chemicals to clean the metal.
Here are some good tips:
- Clean all surfaces before adding coatings or barriers.
- Use non-conductive washers and gaskets between metals.
- Make sure coatings cover all edges and joints.
- Check that sacrificial anodes are in the right spots.
Modern coatings make strong shields against galvanic corrosion by keeping water and salt away. Sacrificial coatings, like zinc on steel, rust first to protect the main part. Using these methods together gives you the best protection.
Environmental Controls
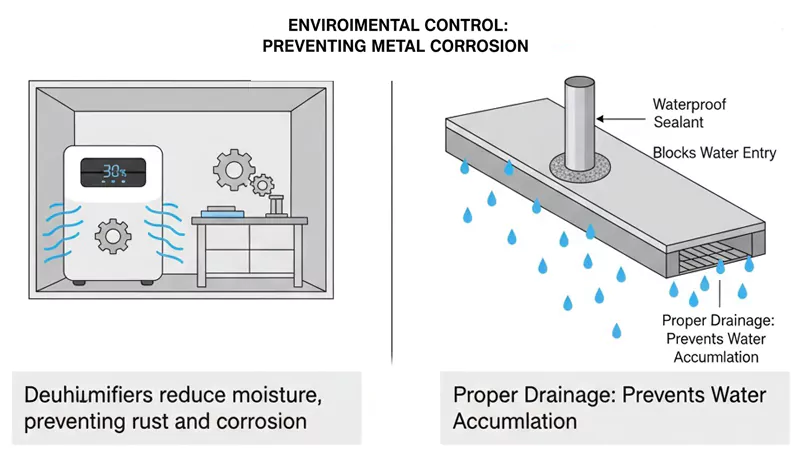
Moisture Management
You can control the area around your metal to slow down corrosion. Moisture is needed for galvanic corrosion to happen. If you keep things dry, it is much harder for corrosion to start. Use dehumidifiers in places with lots of moisture. In marine or tropical areas, this is very important.
You should also design your structures to keep water away from joints. If you can, cover metal parts or use shelters to block rain. Keeping things dry is one of the easiest ways to stop corrosion.
- Control the area by keeping out moisture, humidity, or chemicals.
- Use dehumidifiers in wet or tropical places.
- Build and take care of structures to fight bad weather.
Drainage and Waterproofing
Good drainage is another smart way to protect metal. If water cannot sit on the surface, it cannot cause corrosion. Make sure your structures have sloped surfaces or holes for drainage. This helps water run off fast.
You can also use waterproof materials to seal joints and seams. This keeps water out and stops corrosion before it starts. In concrete, use the right cover and coatings to keep water away from the steel inside.
- Design structures so water does not pool.
- Use waterproofing to seal joints and seams.
- Make sure drains stay clear and working.
By keeping things dry and letting water drain away, you lower the risk of galvanic corrosion. These steps, along with good materials, insulation, and machining, help you stop corrosion and keep your metal strong.
Protective Coatings and Treatments
Protective coatings and treatments help keep metal safe from corrosion. You can use different ways to protect metal, especially where galvanic corrosion might happen. Each way works in its own style to guard the surface and help your equipment last longer.

Paints and Plating
Paints and plating are popular ways to protect metal. Paint makes a wall that keeps water and air away. This stops rust from forming. Powder coating is like paint but uses tiny epoxy bits. These bits melt and stick to the metal, making a hard, strong cover.
Plating puts a thin layer of another metal on your part. For example, you can cover steel with zinc or nickel. This layer keeps out water and air. Galvanizing dips steel in zinc. The zinc layer is a shield that rusts first. If rust starts, the zinc will rust before the steel does.
Here is a table that shows how coatings and treatments work:
| Coating/Treatment | Function | Description |
|---|---|---|
| Paint | Barrier | Stops water and air from touching the metal. |
| Powder Coating | Barrier | Uses epoxy bits for a tough, protective cover. |
| Galvanizing | Sacrificial | Dips steel in zinc, which rusts first to save the steel. |
| Plating | Barrier | Adds a thin layer of metal that does not rust easily. |
| Anodizing | Surface Modification | Makes a thicker oxide layer on metals like aluminum. |
| Passivation | Surface Cleaning | Cleans stainless steel to help stop rust. |
Each way has its own job. Paint and powder coating block water and air. Galvanizing and plating use metals that rust first or do not rust much. Anodizing and passivation change the surface to make it stronger.
- Coatings with metals like zinc and aluminum rust first to protect the main metal.
- These coatings work well in harsh places, like near the sea or in factories.
If you use stainless steel, you can also use passivation. This process cleans the surface and takes away iron. It helps stainless steel fight rust even better.
Anodizing and Passivation
Anodizing and passivation are special ways to treat some metals. Anodizing is best for aluminum. It makes the oxide layer thicker and stronger. This new layer keeps the metal safe from rust and looks nice, too. You can even color anodized aluminum.
Passivation is for stainless steel. It takes away iron from the surface. This makes the chromium layer stronger. When you passivate stainless steel, it fights rust and keeps its shine.
Use anodizing for aluminum parts that need more protection. Use passivation for stainless steel, especially in wet or salty places. Both ways help you stop rust before it starts.
Maintenance and Monitoring
Protective coatings and treatments work best if you take care of them. You need a good plan to check and watch your metal. Regular checks help you find problems early and fix them before they get worse.
Scheduled Inspections
You should make a plan to check your metal structures. Start with a full check when you first put them in. After that, do a full check at least every three years. In wet or salty places, check every year. Use monthly sheets to track small changes. This helps you find little problems before they get big.
- Monthly checks: Look for rust, peeling paint, or color changes.
- Every three months: Check if coatings and anodes are working well.
- Every year: Do a full check of the whole system and fix what is needed.
A table below shows how monitoring helps you:
| Evidence Description | Key Points |
|---|---|
| Corrosion Sensors for Structural Health Monitoring of Oil and Natural Gas Infrastructure: A Review | Real-time checks help you see problems early, keep things safe, and save money on repairs. |
| Limit the Cost of Corrosion Inspection and Repair | Watching all the time lets you plan checks using real data, saving time and money. |
You can use sensors to watch for rust as it happens. This helps you act fast if something changes. Keeping good records makes it easier to plan fixes and avoid surprises.
Early Repairs
When you find a problem, fix it right away. Early repairs stop small problems from getting worse. If you see rust, pits, or peeling paint, clean the spot and put on new coating. Change broken gaskets or anodes as soon as you see a problem.
- Fix small problems as soon as you find them.
- Clean and re-coat spots with damage.
- Change worn-out parts quickly.
By acting fast, you keep your metal strong and safe. You also save money by stopping big repairs later.
Tip: A good plan with regular checks and quick fixes is one of the best ways to keep your metal safe from galvanic corrosion.
Implementing a Corrosion Prevention Plan
A strong corrosion prevention plan helps you keep metal structures safe and long-lasting. You need to look at risks, set up a maintenance schedule, and make sure everyone knows what to do. Good planning stops problems before they start.
Assessing Vulnerability
Start by checking where your metal is most at risk. Walk around your site and look for places where different metals touch, especially if you see stainless steel next to aluminum or copper. Areas that get wet or have salt in the air need extra attention. You should:
- Ask a corrosion expert to help you find weak spots.
- Talk to workers who know the site’s history and past corrosion problems.
- Check records for old repairs or failures.
- Look at joints, bolts, and welds where stainless steel meets other metals.
- Review designs to see if you can use more stainless steel or change how metals connect.
A full site check helps you spot trouble before it grows. If you use stainless steel in the right places, you lower the risk of corrosion.
Maintenance Scheduling
You need a clear schedule to keep your plan working. Regular checks and cleaning help you catch corrosion early. Use this table to guide your maintenance:
| Best Practice | Description |
|---|---|
| Regular Inspections | Check for rust, pitting, or loose coatings, especially on stainless steel. |
| Proper Documentation | Write down what you find and what you fix. |
| Tailored Maintenance | Use special steps for areas with more risk, like near the ocean or where stainless steel meets other metals. |
Other smart steps include:
- Clean surfaces to stop dirt and salt from building up.
- Repaint or recoat as needed, especially on stainless steel parts.
- Use cathodic protection systems to guard against corrosion in pipes or tanks.
- Watch for leaks or water pooling near stainless steel joints.
A good schedule keeps your structures strong and safe.
Training and Documentation
Everyone on your team needs to know how to spot and stop corrosion. Give workers simple guides on what to look for, especially around stainless steel parts. Hold short training sessions to show how to check for rust, pitting, or coating damage. Keep clear records of all checks, repairs, and upgrades.
- Teach staff how cathodic protection works and why it matters.
- Show how to clean and care for stainless steel.
- Keep a logbook for every inspection and repair.
- Update your plan when you add new equipment or change materials.
Tip: When you train your team and keep good records, you make it easier to catch problems early and fix them fast.
A strong corrosion prevention plan uses the right materials, like stainless steel, and smart systems, like cathodic protection. With regular checks, good training, and clear records, you protect your investment and keep your metal structures safe for years.
You can keep metal safe from corrosion by using smart steps.
- First, pick the right materials and plan early.
- Use insulation, sacrificial metals, and coatings together for strong protection.
- Check your metal often and fix problems fast.
- Machining makes surfaces smooth and tight so they do not get damaged easily.
| Expert Tip | Description |
|---|---|
| Ask specialists for help | Talk to experts about your work to find better ways to protect metal. |
Keep learning and stay alert. These steps help you make metal structures that last longer and stay safe.
FAQ
You get galvanic corrosion when two different metals touch and moisture is present. The less noble metal starts to rust faster. This happens most often in wet or salty places.
Look for rust, color changes, or small pits where two metals meet. Paint peeling or strange stains are also signs. Use a flashlight for a closer look.
You cannot always stop it completely, but you can control it. Use compatible metals, add coatings, or install gaskets. Regular checks help you catch problems early.
Machining gives you smooth surfaces and tight fits. Water and dirt have fewer places to hide. This makes it harder for corrosion to start at joints or edges.
Aluminum, steel, and zinc are at higher risk when touching copper or stainless steel. The bigger the difference between the metals, the faster corrosion can happen.
Check at least once a year. In wet or salty areas, check every few months. Use a checklist to track what you find.
Clean the area and remove rust. Reapply coatings or replace damaged gaskets. Fix small problems right away to stop them from getting worse.
Coatings help a lot, but they can wear off or chip. If that happens, corrosion can start again. Always check coatings during inspections.


